Atomic Weights and Isotopic Compositions for All Elements Isotope Relative Atomic Mass Isotopic Composition Standard Atomic Weight Notes: 1: H: 1: 1.007. Atomic weight, also called relative atomic mass, ratio of the average mass of a chemical element ’s atoms to some standard. Since 1961 the standard unit of atomic mass has been one-twelfth the mass of an atom of the isotope carbon-12. Notes on the Atomic Mass of particular elements: Technetium: Atomic mass number given for longest lived isotope. Polonium: Atomic mass number given for longest lived isotope. Hydrogen -1 Helium-4 Lithium = 7 Beryllium = 9 Boron = 11 Carbon = 12 Nitrogen = 14 Oxygen = 16 Fluorine = 19 Neon = 20 Sodium = 23 Magnesium = 24 Aluminum = 27 Silicon = 28 Phosphorus =31 Sulfur = 32 Chlorine =35 Argon = 40 Potassium = 39 Calcium.
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.
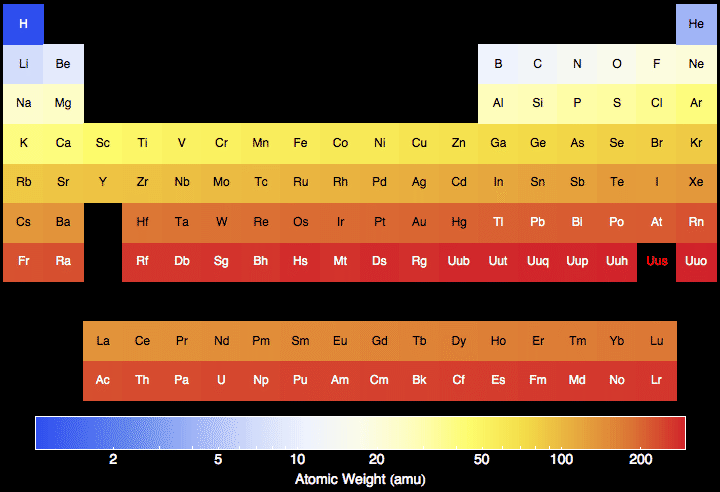
Samsung m2070 scan software mac. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below.
Note:
%2C_black_and_white.png?revision=2)
- The Atomic masses are represented in the Atomic mass unit (u).
- The elements whose atomic masses are written in bracket ( ) are the synthetic elements and their atomic masses values represent the Atomic Mass of the most stable isotope.
Bonus Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro. Earn to die 2spiter games.
3). Game 208: june 15, 2018the initials game. You will also get the HD images of the Periodic table (for FREE).
Atomic Mass 9

What Is The Atomic Mass Of All Elements
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
